Eric J. Topol, MD: Hello. I'm Eric Topol, editor-in-chief of Medscape. It's a real delight to welcome John Rogers from Northwestern University in Evanston, Illinois. John is probably the world leader in developing creative sensors that interact with the human body. Welcome, John.
John A. Rogers, PhD: It is a pleasure to be here.
Dr Topol: You went to the University of Texas at Austin, and then on to the Massachusetts Institute of Technology (MIT), right?
Dr Rogers: Yes. I was at MIT for 6 years. I got master's degrees in physics and chemistry, and then a PhD in physical chemistry. After that, I got a junior fellowship at the Harvard University Society of Fellows, which was like a super-post-doc, a 3-year position. The appeal was that it provided a salary and a stipend, and you could sort of do whatever you wanted.
I ended up splitting my time between work in the Harvard laboratory of George Whitesides, a famous materials chemist and entrepreneur, with the other half spent with a start-up company that we launched out of MIT based on my PhD research. That was a really fun time, from 1995 through 1997.
Dr Topol: Then you went to Bell Labs?
Dr Rogers: Yes. I interviewed for a number of positions, but I was always drawn to the interface between science and technology. Bell Labs in those days was like the New York Yankees of science, with its focus on materials, technology, lasers, and integrated circuits. I spent 5 great years there, during which I was really able to expand my areas of expertise.
That "Bell Labs–ian" mindset has really stayed with me and shaped my research. After Bell Labs, I went to the University of Illinois at Urbana.
'Epidermal Electronics': Thin Skin Sensors
Dr Topol: I first learned of you from a 2011 paper in Science[1] about "skin chips," which you pioneered. Tell us a bit about that.
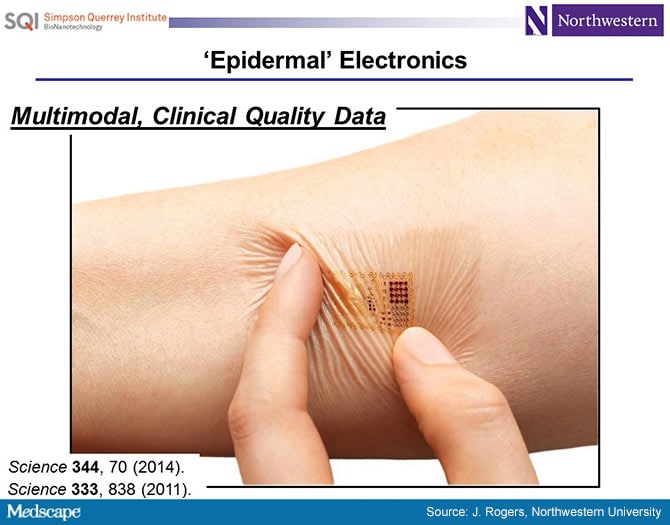
Dr Rogers: That was a really important paper for us. My core expertise is in electronic materials. Most people who are doing academic research in electronic materials are thinking about a future of electronics driven by Moore's Law.
Transistors are getting smaller and smaller; microprocessors have more and more transistors per unit area, at higher and higher clock speeds, with more and more sophistication in their operation. That is a really compelling area for research, because to enable a continuation of Moore's Law will require a lot of scientific discovery and new academic insights into materials at those scales.
We have been interested in a different future of electronics, not so much driven by the critical dimensions of transistors, but thinking about electronics as a possible interface to the human body. Here, you need to consider things around materials biocompatibility and, maybe more important, physical properties that enable an intimate integration of circuit technology with the soft-textured, time-dynamic surfaces that you find in biology. It's quite a bit different from anything that has ever been present in commercial forms of integrated circuits, which were all built in the rigid planar surfaces of semiconductor wafers.
This paper was the first demonstration of all the key ideas around the material science, the mechanics' designs, the device architectures, the system-level considerations that allowed us to create skin-like forms of electronics.
Dr Topol: I remember the pictures from the Science paper, with these little skin chips on the forehead for an electroencephalogram and on the chest for a cardiogram, and thinking that this was mind-blowing.
What I do not understand is how you moved from this engineering background with chemistry and physics into wanting to deal with the body and coalescing chips with it.
Dr Rogers: It's an interesting story. We got into this whole area of flexible electronics when I was back at Bell Laboratories, where we were developing new classes of semiconductor materials. The idea was that if you could create integrated circuits on sheets of plastic, you might be able to build new classes of consumer gadgets. In particular, we were interested in paper-like flexible displays.
I was giving a talk in electrical engineering at the University of Pennsylvania on this topic and some neuroscientists came up to me afterwards. They said, "Hey, these are cool for large-area steerable antennas for the military or flexible displays for consumer applications, but have you ever thought about putting these things on the brain? That would be really interesting, because you could map electrical activity on the brain."
I had never really thought about that bio interface until that time, and it struck me as a huge opportunity. So, we started collaborations on that basis. We were also interacting with cardiologists from the University of Pennsylvania Medical School. Understanding epilepsy also turned out to be an area of focus, using these sheets as diagnostic platforms.
This was a direction I wanted to pursue, but as you know, it's ultimately the students who have a large impact on your research. And this was such a compelling idea for them. They saw a much greater and higher purpose in working on technologies that could have a positive impact on human healthcare than technologies that could be used for a new consumer electronics gadget. So it was those two things that came together.
Dr Topol: The things you previously developed were already mind-blowing, but since then, you've developed the Snapchat of chips in the body.
Dr Rogers: I have never heard it referred to that way! (laughing) That's pretty good!
Dr Topol: It disintegrates, dissolves, and self-destructs. It's incredible! Then you had all of these other ideas to use these chips on the brain and in the heart, catheters with chips, testing for things like hydration or blood pressure. Is there anything you cannot measure with these chips?
Dr Rogers: That is a good question. Most of the sensors that we focused on up to this point have really emphasized physical property measurements, such as strains, stresses, electrical potential, temperature, and things like that. I think the frontier is in biochemical rather than biophysical measurements.
Sweat: The 'Holy Grail'
Dr Topol: You published a Science Translational Medicine article[2] that featured this very cool design for a sweat sensor that can measure lactate and electrolytes, which obviously was a stunner as well, but where can that go? Do you have to have actual sweat or can you just get that right on the skin?
Dr Rogers: Right now, we are just capturing sweat that is emerging from the skin in physical liquid form. The eccrine glands are acting as pumps to push that sweat into these microfluidic analysis networks. We can do a lot with that once we have even just microliter volumes of sweat; we can route it around and do all kinds of analysis.
But, as you know, your body is constantly undergoing sweating without enough liquid volume to form well-defined droplets, and that evaporates almost immediately. We think there is a great opportunity for new device designs and materials that can capture that insensible sweat, so you do not have to be physically exercising in order to capture this kind of bio fluid. That's an area of ongoing research for us and it's tantalizing.
Dr Topol: Do you think you'll be able to do it?
Dr Rogers: It seems like it should be possible. If you look at your skin under a microscope, you can see that your sweat glands are constantly firing. There is liquid there, but it immediately evaporates. Right now we can capture the water vapor associated with insensible sweat and condense it in our devices. So we thought we had the problem solved, but unfortunately the evaporation process leaves all the biomarkers behind.
Dr Topol: If you can do that, it will be like the Holy Grail, as all of these things correlate so well with what is in the blood. That will be a big one.
You just moved to Northwestern University a year ago, and what I learned just today is that you have taken these chips, which are no longer just fascinating breakthroughs but are now in infants, in kids, in intensive care units. Tell us about that.
Dr Rogers: That was one of the primary motivations in moving from the University of Illinois, where I spent 13 very productive years with great colleagues. However, there is no medical school in Urbana.
We had been doing pretty well collaborating with medical schools at different universities, in St. Louis, continuing our work with the University of Pennsylvania Medical School, and so on. But we perceived greater opportunities being geographically co-located with an active medical school with researchers there.
The move to Northwestern has really blown out the number of interactions we have with clinicians. It's great to publish papers and educate the graduate students and so on, but we are defining success by proliferation of the technologies. The best, most effective way to do that is to get engaged very intimately with a medical school.
- 10
- References
Medscape © 2018 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: The Future of Medicine From a Leader in Biosensors - Medscape - Mar 15, 2018.









