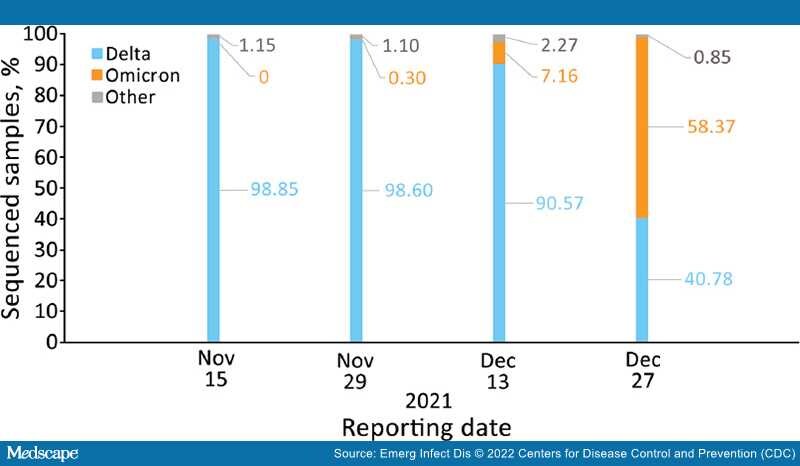
Methods
Study Design
We conducted a retrospective longitudinal cohort study using 2 MOH national repositories: the COVID-19 vaccine repository and the SARS-CoV-2 test repository. The national COVID-19 vaccine repository includes vaccine type, vaccine lot number, and date of dose administration for each person vaccinated in Israel. The national SARS-CoV-2 PCR test database includes the results of each test performed, the date of testing, and the date results were obtained for each person. It also includes the date of hospitalization, severity of illness, and date of death of persons with COVID-19, if applicable. Personal identifiers such as unique personal identity number, age, and sex of each person registered in the repositories (because of PCR testing or vaccination) are included in both databases. We retrieved individual deidentified data from both databases and matched persons by using twice-encrypted unique personal identity numbers.
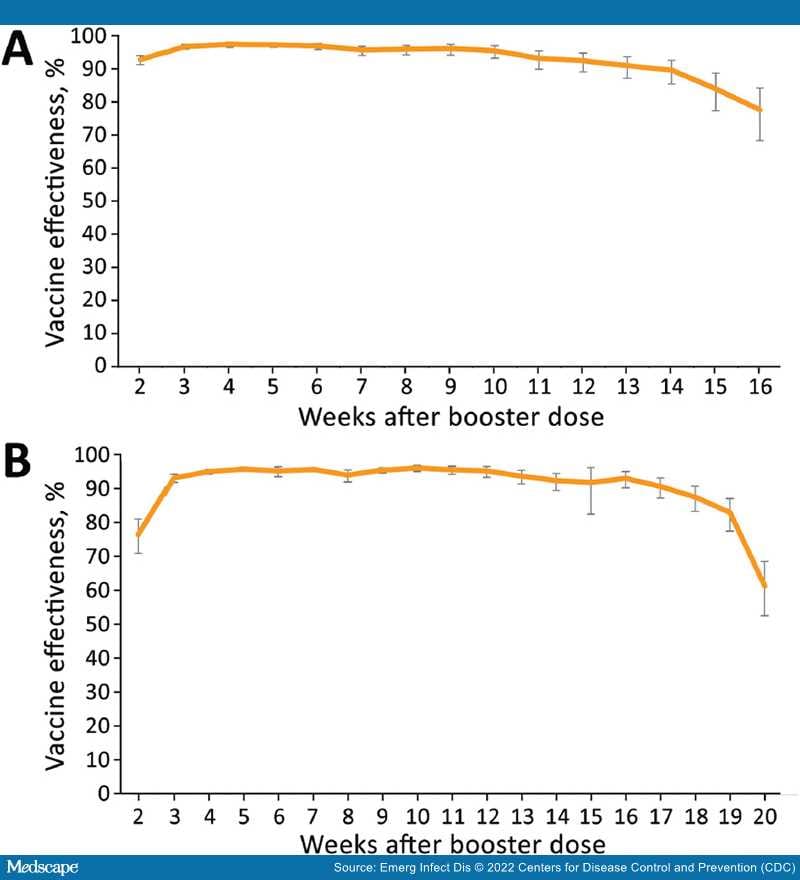
During the first stage of our study, we determined VE for booster dose vaccine recipients against SARS-CoV-2 infection by using unvaccinated persons as controls. During the second stage, we determined the rate reduction for hospitalizations, severe or critical disease, and deaths among persons who tested positive for SARS-CoV-2 after the booster dose (i.e., breakthrough cases).
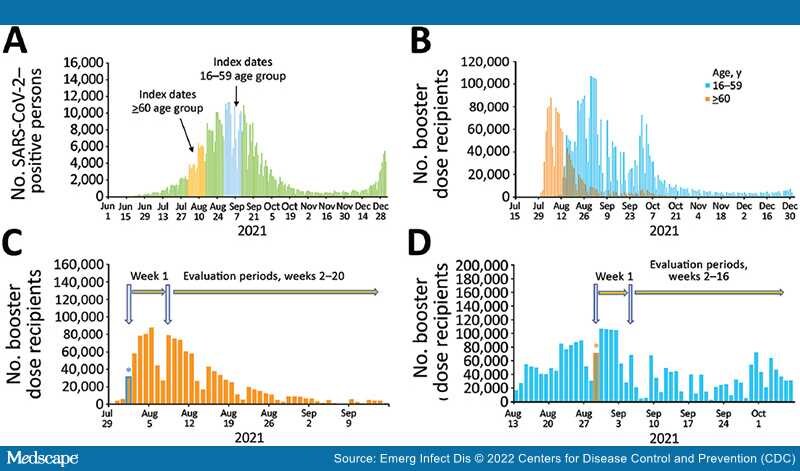
We defined as index dates the dates on which third-dose vaccine recipients in our study received the booster dose (Figure 1, panel A).