Abstract and Introduction
Abstract
Background: Intravenous (IV) golimumab, a TNFi, is approved for treating rheumatoid arthritis (RA), psoriatic arthritis (PsA), and ankylosing spondylitis (AS). We analyzed pooled safety results from three phase 3 IV golimumab trials in these rheumatologic diseases and hypothesized that the safety profile of IV golimumab would be similar to that established for other TNFi, including subcutaneous golimumab.
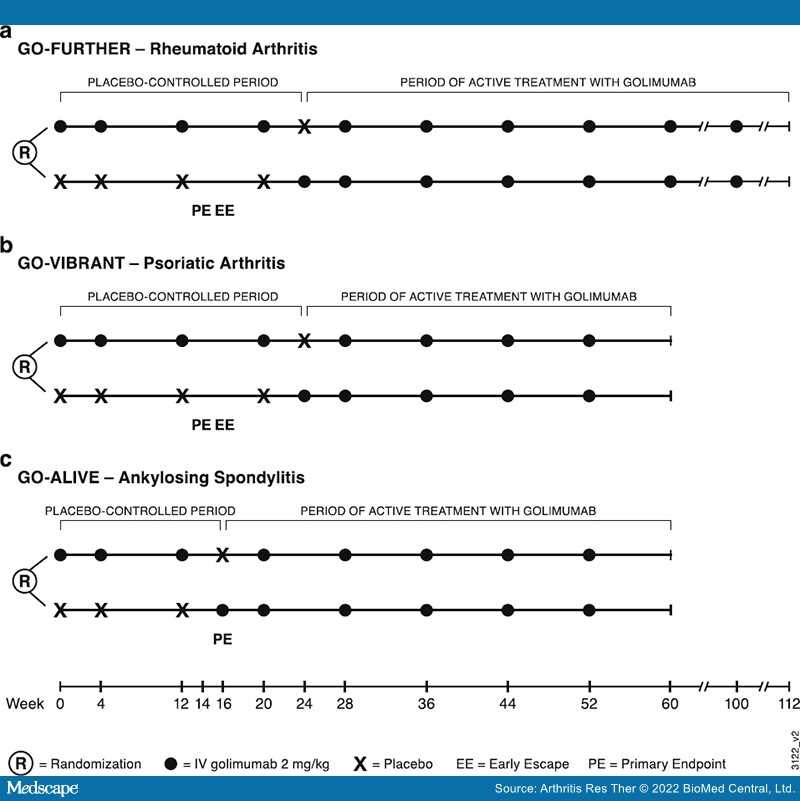
Methods: Data from three double-blind, randomized trials of IV golimumab in patients with RA, PsA, and AS, each with a placebo-controlled period and an extension of active treatment, were included. Golimumab 2 mg/kg was administered at weeks 0 and 4, then every 8 weeks through week 100 (RA) or week 52 (PsA, AS). Concomitant low-dose, oral corticosteroids were permitted. Concomitant methotrexate was required in the RA trial and permitted in the PsA and AS trials; placebo patients crossed over to golimumab at weeks 24 (RA, PsA) and 16 (AS), respectively. Adverse events (AEs), including infections, serious infections, malignancies, and major adverse cardiovascular events (MACE), were assessed through week 112 (RA) or week 60 (PsA, AS).
Results:In total, 539 patients were randomized to placebo, and 740 patients were randomized to golimumab; 1248 patients received ≥ 1 golimumab administration. Among the placebo and golimumab patients, respectively, during the placebo-controlled periods, 40.6% and 50.3% had an AE, 2.4% and 3.8% had a serious AE, and 0.4% and 0.8% had a serious infection. Among all golimumab-treated patients, the numbers of events/100 patient-years (95% CI) were as follows: AEs, 175.2 (169.0, 181.6); serious AEs, 12.7 (11.0, 14.5); serious infections, 3.4 (2.5, 4.4); active tuberculosis, 0.4 (0.1, 0.8); opportunistic infection, 0.2 (0.1, 0.6); malignancies, 0.4 (0.2, 0.9), and MACE, 0.5 (0.2, 1.0). There were no cases of lymphoma. Three (0.6%) placebo-treated patients and 6 (0.5%) golimumab-treated patients died during the studies. Concomitant methotrexate was associated with increased occurrence of elevated alanine transaminase levels and lower incidence of antibodies to golimumab. During the placebo-controlled periods, serious infections in the placebo and golimumab groups were more common in patients receiving concomitant low-dose oral corticosteroids vs. those not receiving corticosteroids.