Abstract and Introduction
Abstract
Graphical Abstract
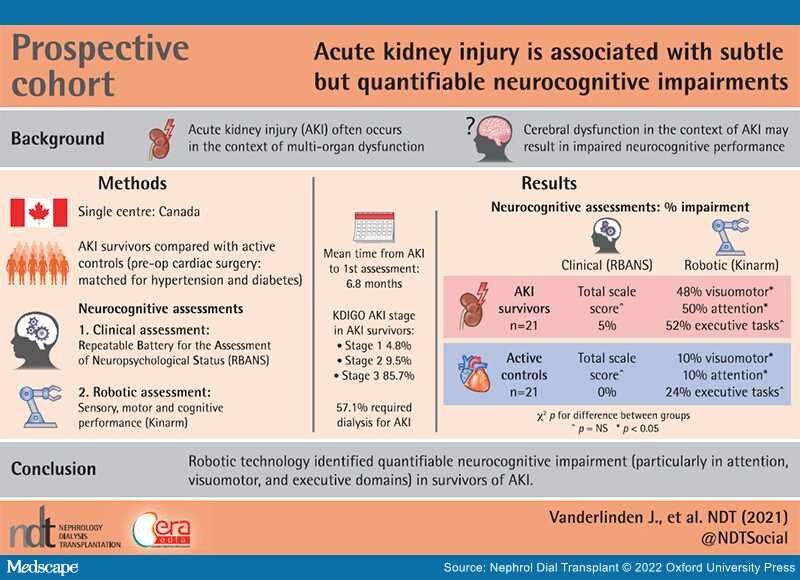
Background: Acute kidney injury (AKI) is associated with long-term morbidity and mortality. The effects of AKI on neurocognitive functioning remain unknown. Our objective was to quantify neurocognitive impairment after an episode of AKI.
Methods: Survivors of AKI were compared with age-matched controls, as well as a convenience sample of patients matched for cardiovascular risk factors with normal kidney function (active control group). Patients with AKI completed two assessments, while the active control group completed one assessment. The assessment included a standardized test: the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), and a robotic assessment: Kinarm.
Results:The cohort consisted of 21 patients with AKI, 16 of whom completed both assessments, and 21 active control patients. The majority of patients with AKI had Kidney Disease: Improving Global Outcomes Stage 3 AKI (86%), 57% received dialysis and 43% recovered to ≤25% of their baseline serum creatinine by their first assessment. Compared with the RBANS, which detected little impairment, the Kinarm categorized patients as impaired in visuomotor (10/21, 48%), attention (10/20, 50%) and executive tasks (11/21, 52%) compared with healthy controls. Additionally, patients with AKI performed significantly worse in attention and visuomotor domains when compared with the active controls.