Abstract and Introduction
Abstract
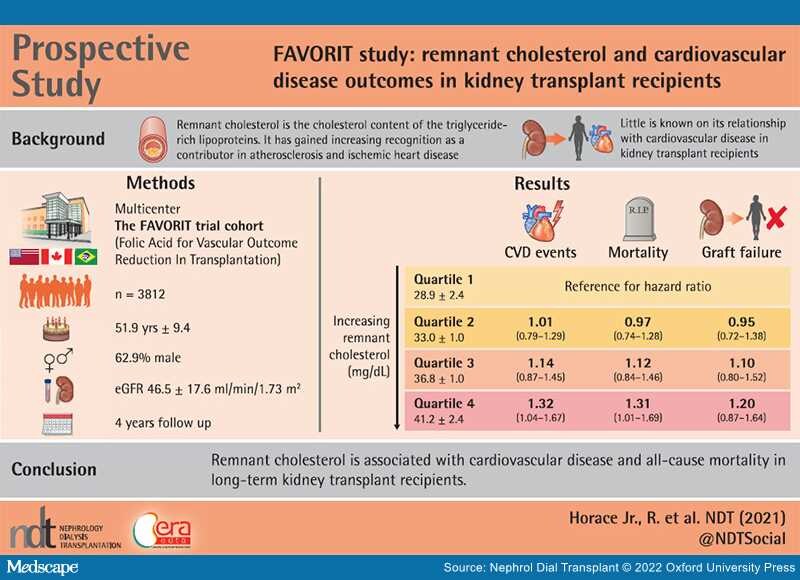
Graphical Abstract
Background: The cholesterol content of circulating triglyceride-rich lipoproteins is characterized as remnant cholesterol, although little is known about its role in the development of cardiovascular disease (CVD) outcomes, all-cause mortality or transplant failure in kidney transplant recipients (KTRs). Our primary aim was to investigate the prospective association of remnant cholesterol and the risk of CVD events in renal transplant recipients with secondary aims evaluating remnant cholesterol and renal graft failure and all-cause mortality among participants in the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) trial.
Methods: Among 4110 enrolled participants, 98 were excluded for missing baseline remnant cholesterol levels and covariates. Nonfasting remnant cholesterol levels were calculated based on the lipid profiles in 3812 FAVORIT trial participants at randomization. A Wilcoxon-type test for trend was used to compare baseline characteristics across remnant cholesterol quartiles. Cox proportional hazards regression was used to evaluate the association of baseline remnant cholesterol levels with time to primary and secondary study outcomes.
Results:During a median follow-up of 4.0 years we documented 548 CVD incident events, 343 transplant failures and 452 all-cause deaths. When comparing the highest quartile (quartile 4) to quartile 1, proportional hazard modeling revealed a significant increase in CVD risk {hazard ratio [HR] 1.32 [95% confidence interval (CI) 1.04–1.67]} and all-cause mortality risk [HR 1.34 (95% CI 1.01–1.69)]. A nonsignificant increase in transplant failure was seen as well [HR 1.20 (95% CI 0.87–1.64)].