Abstract and Introduction
Abstract
Nonsteroidal anti-inflammatory drugs are among the most commonly administered drugs in the perioperative period due to their prominent role in pain management. However, they potentially have perioperative consequences due to immune-modulating effects through the inhibition of prostanoid synthesis, thereby affecting the levels of various cytokines. These effects may have a direct impact on the postoperative outcome of patients since the immune system aims to restore homeostasis and plays an indispensable role in regeneration and repair. By affecting the immune response, consequences can be expected on various organ systems. This narrative review aims to highlight these potential immune system–related consequences, which include systemic inflammatory response syndrome, acute respiratory distress syndrome, immediate and persistent postoperative pain, effects on oncological and neurologic outcome, and wound, anastomotic, and bone healing.
Introduction
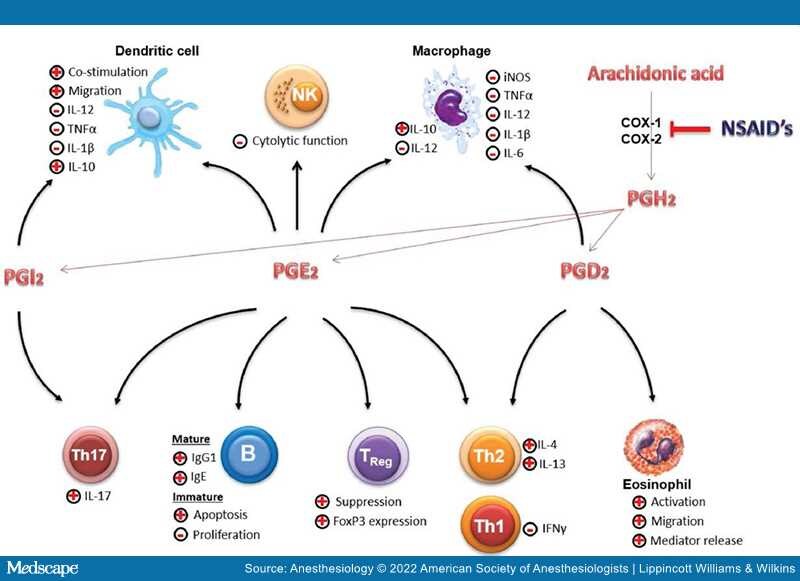
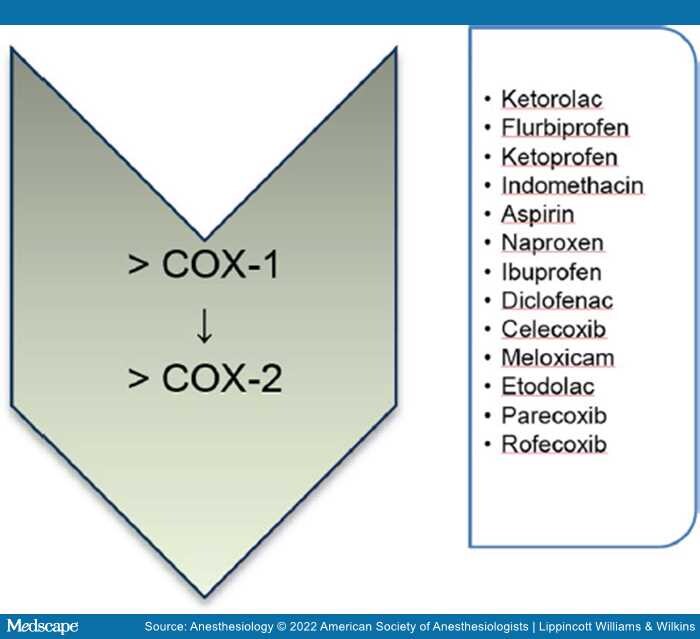
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used and are known for their analgesic, antipyretic, and immune-modulating properties. Their mechanism of action is though inhibition of cyclooxygenase, also known as prostaglandin–endoperoxide synthase, the key enzyme involved in prostaglandin synthesis. Cyclooxygenases (COX-1 and COX-2) catalyze the formation of prostaglandin (PS) H2 (PGH2) from arachidonic acid, upon which PGH2is metabolized by tissue-specific isomerases to other prostanoids such as prostaglandins (PGD