Abstract and Introduction
Abstract
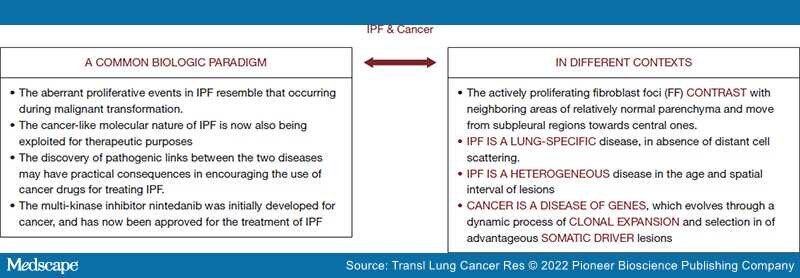
Background and Objective: Translational research is a source of continuous innovation in medicine, more particularly for clinical research on new treatment modalities in idiopathic pulmonary fibrosis (IPF) patients. However, the heterogeneity of the disease is well recognized, and different pathological and molecular settings have been identified. The molecular mechanisms by which IPF proceeds in time and space remains poorly understood. Although some IPF features are reminiscent of cancer, the dynamics of malignant divergent clonal selective pressure and heterogeneity clearly differ from those occurring in IPF. This is reflected in the absence of patient proper selection and stratification to biological agents (pirfenidone, nintedanib) which limit therapeutic efficacy. Consequently, increased costs are related to the clinical management of advanced IPF patients. Steady collaboration and fluid communication between pneumo-oncologists, radiologists and molecular biologists is a clear priority for the correct interpretation of tests and the definition of effective personalized strategies against this orphan disease. The present work aims at providing the most relevant hints shared by cancer and IPF.
Methods:A systematic literature review was performed to identify all relevant data. The examined databases were Scopus, Web of Science, Cochrane, Google Scholar, and PubMed. The last search was run on January 5, 2022.