Abstract and Introduction
Abstract
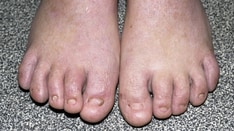
Epidermolysis bullosa (EB) encompasses a heterogeneous group of inherited skin fragility disorders, with mutations in genes encoding the basement membrane zone (BMZ) proteins that normally ensure dermal–epidermal integrity. Of the four main EB types, recessive dystrophic EB (RDEB), especially the severe variant, represents one of the most debilitating clinical entities, with recurrent mucocutaneous blistering and ulceration leading to chronic wounds, infections, inflammation, scarring and ultimately cutaneous squamous cell carcinoma, which leads to premature death. Improved understanding of the molecular genetics of EB over the past three decades and advances in biotechnology have led to rapid progress in developing gene and cell-based regenerative therapies for EB. In particular, RDEB is at the vanguard of advances in human clinical trials of advanced therapeutics. Furthermore, the past decade has witnessed the emergence of a real collective, global effort involving academia and industry, supported by international EB patient organizations such as the Dystrophic Epidermolysis Bullosa Research Association (DEBRA), among others, to develop clinically relevant and marketable targeted therapeutics for EB. Thus, there is an increasing need for the practising dermatologist to become familiar with the concept of gene therapy, fundamental differences between various approaches, and their human applications. This review explains the principles of different approaches of gene therapy, summarizes its journey, and discusses its current and future impact in RDEB.