Abstract and Introduction
Abstract
Precipitated by chronic psychological stress, immune system dysregulation, and a hyperinflammatory state, the sequelae of postacute COVID-19 (long COVID) include depression and new-onset diabetes. We hypothesize that exercise counters the neuropsychiatric and endocrine sequelae of long COVID by inducing the release of circulating factors that mediate the anti-inflammatory response, support brain homeostasis, and increase insulin sensitivity.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the pathogen that causes coronavirus disease 2019 (COVID-19) and has contributed to millions of deaths globally. In some cases, persistent symptoms and development of sequelae occur 4 to 12 wk after onset of acute COVID-19 symptoms (long COVID).[1] The antibody response is consistent with durable immunity against secondary COVID-19 disease.[2] Nevertheless, SARS-CoV-2 mRNA and protein are active in the small intestinal epithelium of some individuals nearly 6 months after a COVID-19 diagnosis.[3]
The underlying pathophysiology of COVID-19 is multifaceted, and the components seem inextricably linked. The variability of the clinical disease trajectories in patients with COVID-19 is marked by disparities that outweigh commonalities.[1] Therefore, understanding the cellular mechanisms and critically evaluating convergence among observations become necessary to arrive at an informed strategy for managing the risk of long COVID and preventing its escalation.
SARS-CoV-2 bind to the ACE2 receptor expressed on pancreatic β cells and induce cell damage that can worsen preexisting diabetes or precipitate the onset of diabetes.[4,5] Diabetic ketoacidosis typically observed in type 1 diabetes, which is an autoimmune condition, occurs in patients without a preexisting diabetes diagnosis weeks to months after resolution of COVID-19. Cytokines such as interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) are elevated in patients with severe COVID-19.[1]
Physical inactivity is associated with an increased risk for development of type 2 diabetes and more severe outcomes from COVID-19. The odds that a physically inactive person will encounter severe COVID-19 outcomes exceed that of most chronic diseases.[6] We hypothesize that exercise promotes the release of circulating mediators that are central to attenuation of the long-term neuroendocrine symptoms of COVID-19 (Figure 1). In this review, we present biological insights into maladaptive stress patterns that predispose individuals to clinical depression and glucose dysregulation characteristic of type 2 diabetes. We evaluate the evidence to support our testable hypothesis that exercise can prevent or mitigate the long-term sequelae of COVID-19.
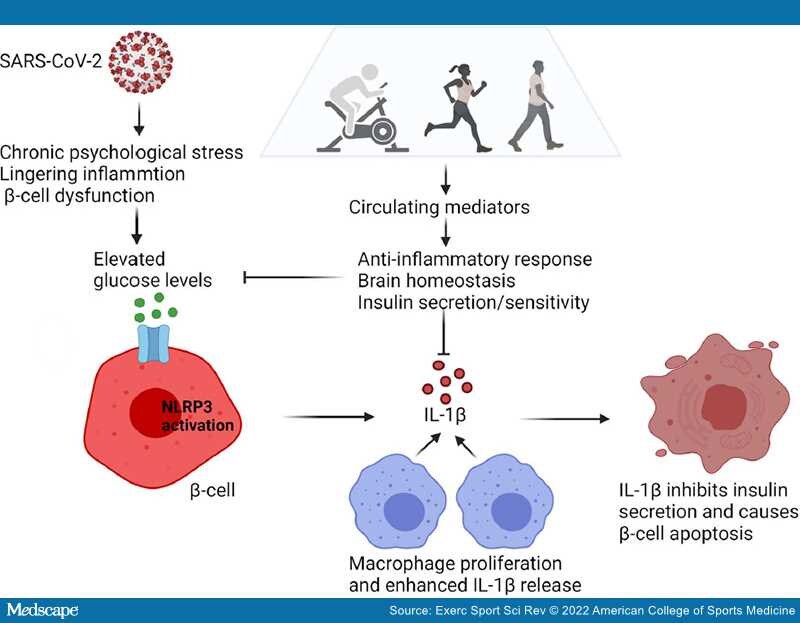
Figure 1.
The development of hyperglycemia arising from disruption of immune metabolic homeostasis in COVID-19. High glucose levels induced by psychological stress, lingering inflammation, and β-cell dysfunction can lead to activation of the NLRP3 inflammasome in pancreatic β cells. As a result, pro–IL-1β is processed to the biologically active IL-1β. IL-1β released from β cells causes the recruitment and activation of macrophages, which prompts the release of more IL-1β. High local concentrations of IL-1β in the β-cell microenvironment may inhibit insulin secretion and trigger β-cell dysfunction and apoptosis. This leads to further increases in levels of glucose, thereby causing IL-1β autostimulation and establishing a vicious cycle. Exercise induces the release of circulating factors that mediate the anti-inflammatory response, support brain homeostasis, and increase insulin sensitivity. The net effect is the lowering of glucose levels and could be envisioned as a remission-induction therapy to counter the sequelae of COVID-19 (graphics program: Biorender). IL-1β, Interleukin-1β; NLRP3, NOD-, LRR-, and pyrin domain-containing protein 3.
Exerc Sport Sci Rev. 2022;50(2):65-72. © 2022 American College of Sports Medicine