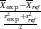Abstract and Introduction
Abstract
The US FDA issued a black-box warning against co-prescription of antipsychotic (AP) agents and opioids due to the risk of respiratory depression, but evidence on the comparative safety of sedating vs nonsedating APs is lacking. We classified APs as sedating (eg, quetiapine, olanzapine, and chlorpromazine) and nonsedating (eg, aripiprazole, haloperidol, and risperidone) based on their affinity to the histamine-1 neuroreceptor (Ki < or ≥20, respectively) and sought to compare the rate of overdose between patients using sedating vs nonsedating APs plus opioids. We constructed a population-based cohort nested in the IBM MarketScan database (2004–2017). Patients with concomitant use of sedating APs and prescription opioids ("exposed") were 1:1 matched to patients with concomitant use of nonsedating APs and prescription opioids ("referent") based on the propensity score (PS). The primary outcome was any hospitalization or emergency department visit due to an overdose within 30 days. The final cohort comprised 62 604 exposed and an equal number of PS-matched reference patients. Characteristics of matched exposed and reference patients were similar. There were 178 overdose events among the exposed (35.3 events per 1000 person-years [PY]) vs 133 among the reference group (26.4 events per 1000 PY), for an adjusted hazard ratio of 1.34 (95% CI: 1.07–1.68). This finding was consistent across sensitivity and subgroup analyses. Among patients receiving prescription opioids, concomitant use of sedating APs was associated with an increased risk of overdose compared with nonsedating APs. Caution is required when co-prescribing opioids and APs. If co-prescription is needed, choosing a nonsedating agent should be preferred whenever possible given the clinical context.