A new film called Salt in My Soul was recently released, with one of the expressed goals being to raise funds for phage research and antibiotic resistance.

Mallory Smith
The film shares the story of Mallory Smith, a young woman, now deceased, who suffered from cystic fibrosis. She kept video and written diaries that she shared with her family only after her death from a multidrug-resistant Burkholderia cepacia infection. In a desperate attempt to save her, Mallory received phage therapy. Her autopsy showed that the phages had begun to work, killing the B cepacia as intended. They just hadn't had enough time.
Phage History
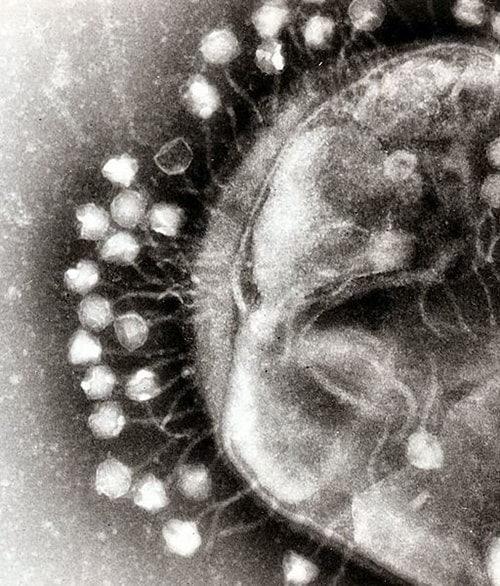
The name bacteriophage is an apt description. Its Greek origin means "bacteria eater." Phages are viruses that infect bacteria; many cause the bacteria to lyse (disintegrate). Discovered in 1915 by French-Canadian microbiologist Felix d'Herelle, phages were used briefly to control cholera outbreaks. In 1934, d'Herelle helped establish a phage research institute with fellow microbiologist George Eliava in the Eastern European nation of Georgia (now known as the Eliava Institute of Bacteriophages, Microbiology and Virology). Phage therapies were said to have been employed by the German army during World War II as well as in the Soviet Unionand Japan during that era.










