Abstract and Introduction
Introduction
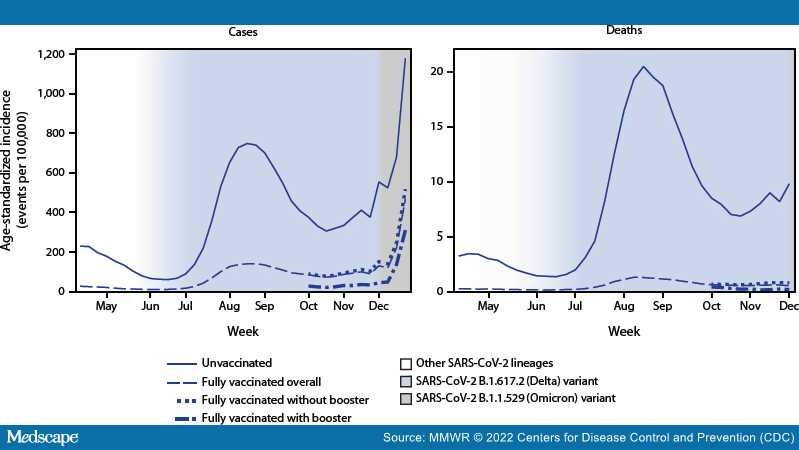
Previous reports of COVID-19 case, hospitalization, and death rates by vaccination status† indicate that vaccine protection against infection, as well as serious COVID-19 illness for some groups, declined with the emergence of the B.1.617.2 (Delta) variant of SARS-CoV-2, the virus that causes COVID-19, and waning of vaccine-induced immunity.[1–4] During August–November 2021, CDC recommended§ additional primary COVID-19 vaccine doses among immunocompromised persons and booster doses among persons aged ≥18 years.[5] The SARS-CoV-2 B.1.1.529 (Omicron) variant emerged in the United States during December 2021[6] and by December 25 accounted for 72% of sequenced lineages.[7] To assess the impact of full vaccination with additional and booster doses (booster doses),¶case and death rates and incidence rate ratios (IRRs) were estimated among unvaccinated and fully vaccinated adults by receipt of booster doses during pre-Delta (April–May 2021), Delta emergence (June 2021), Delta predominance (July–November 2021), and Omicron emergence (December 2021) periods in the United States. During 2021, averaged weekly, age-standardized case IRRs among unvaccinated persons compared with fully vaccinated persons decreased from 13.9 pre-Delta to 8.7 as Delta emerged, and to 5.1 during the period of Delta predominance. During October–November, unvaccinated persons had 13.9 and 53.2 times the risks for infection and COVID-19–associated death, respectively, compared with fully vaccinated persons who received booster doses, and 4.0 and 12.7 times the risks compared with fully vaccinated persons without booster doses. When the Omicron variant emerged during December 2021, case IRRs decreased to 4.9 for fully vaccinated persons with booster doses and 2.8 for those without booster doses, relative to October–November 2021. The highest impact of booster doses against infection and death compared with full vaccination without booster doses was recorded among persons aged 50–64 and ≥65 years. Eligible persons should stay up to date with COVID-19 vaccinations.