Results
Sample Characteristics and Demographics
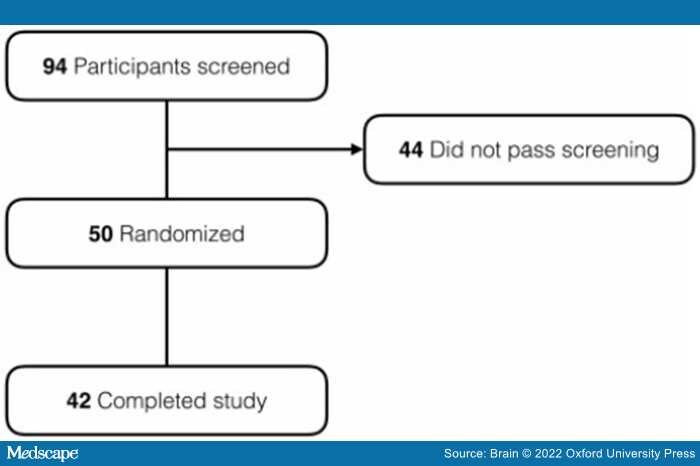
A total of 94 participants were screened at the two performance sites, of which 44 did not meet inclusion/exclusion criteria. The remaining 50 participants were randomly assigned to receive riluzole (n = 26) or placebo (n = 24). Of these, 22 patients receiving riluzole and 20 patients receiving placebo completed the study and had both FDG-PET time points. The diagram in Figure 2 shows the participant disposition.
Figure 2.
Enrolment, randomization and trial completion.
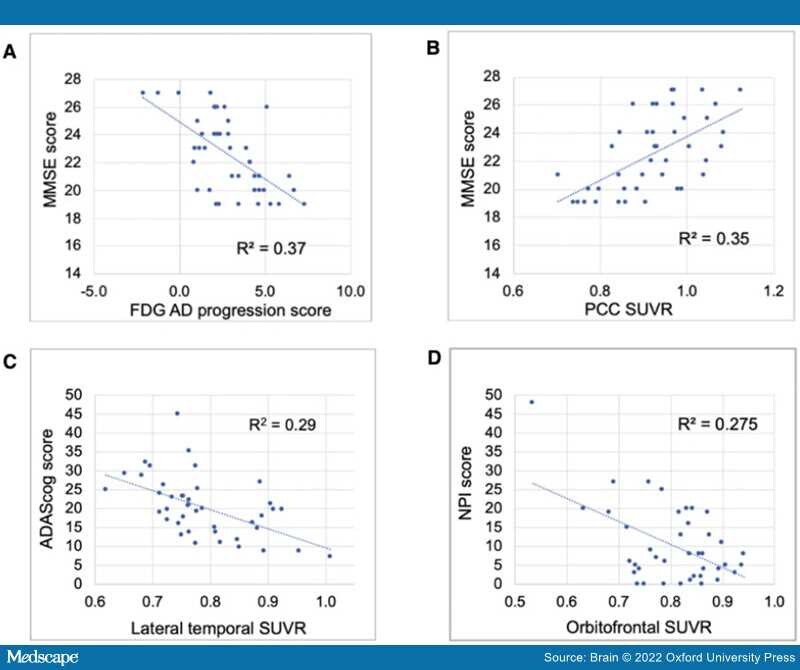
Enrolled participants comprised 26 females and 16 males, aged 58 to 88, and 58% were apolipoprotein 4 (APOE ɛ4) carriers (Table 1, one participant unavailable). There were no significant between-group differences in baseline characteristics of the patients with respect to age, sex, education or APOE ɛ4, although there was a trend for a greater proportion of APOE ɛ4 carriers in the riluzole group. Baseline neuropsychological measures were well balanced for MMSE, NPI, ADL total, Clinical Dementia Rating scale total in riluzole group in comparison to placebo; however, the riluzole group trended as more impaired in ADAS-cog than placebo at baseline (P = 0.08; Table 1).
Neuroimaging Outcome Measures
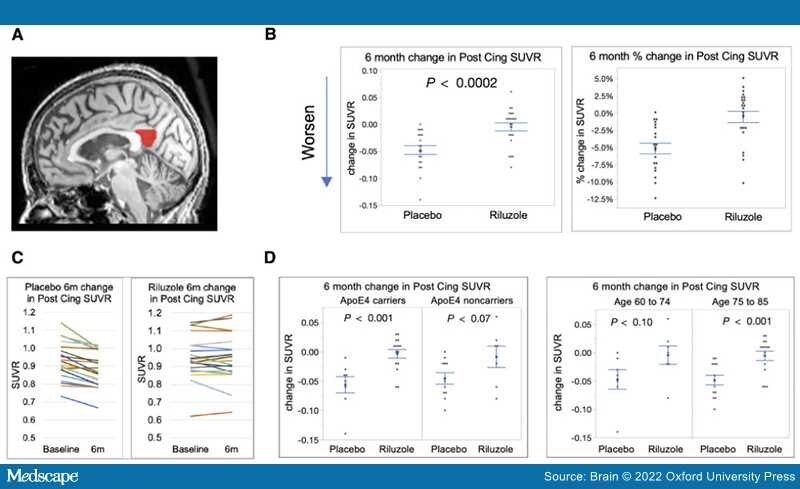
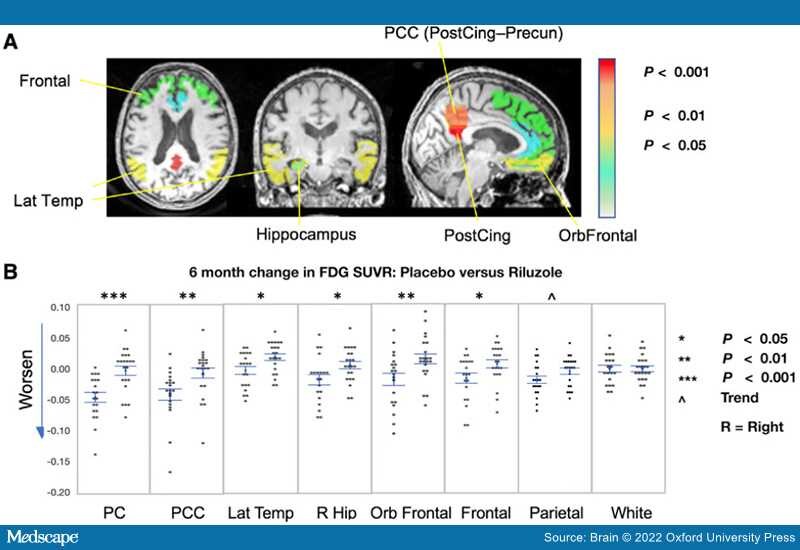
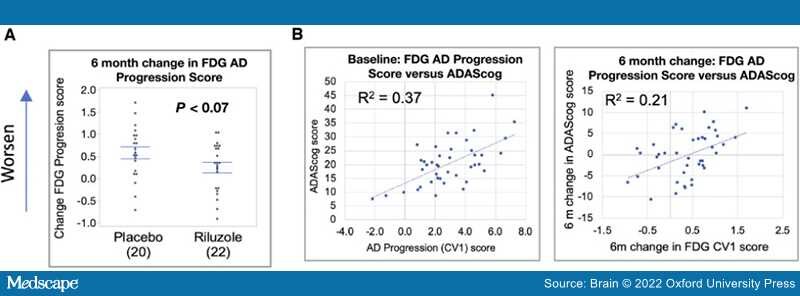
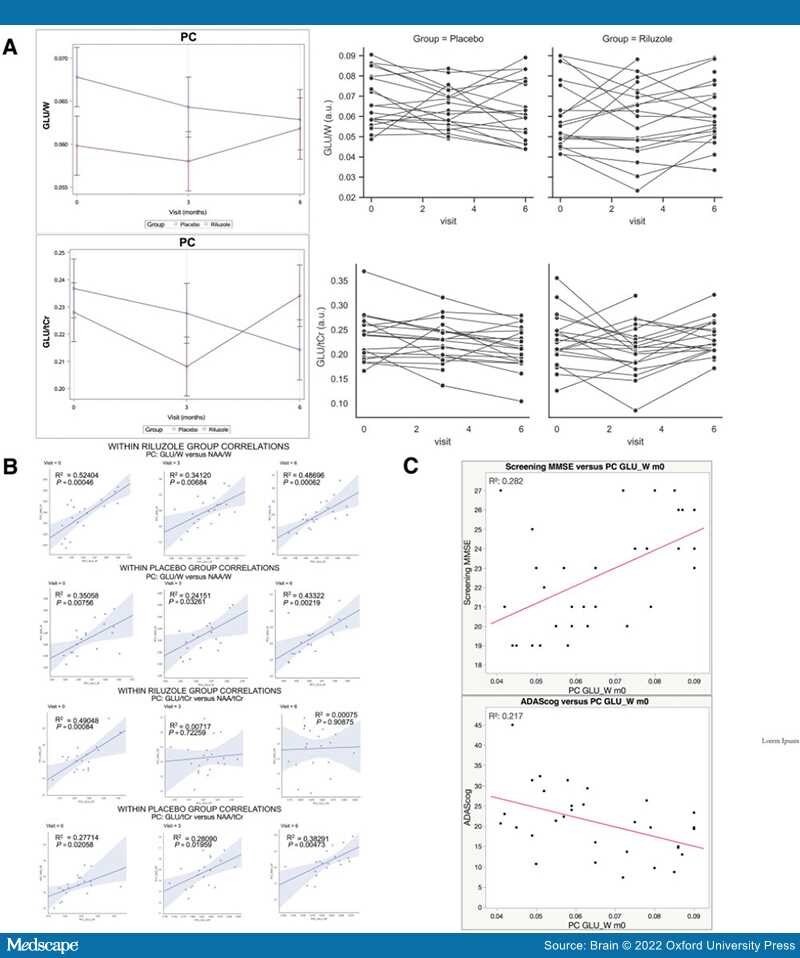
FDG-PET.The study's main primary outcome measure confirmed a difference between arms in FDG-PET cerebral metabolic changes over the 6-month treatment period, with less decline in multiple prespecified brain regions in the riluzole group in comparison to placebo group.