Abstract and Introduction
Introduction
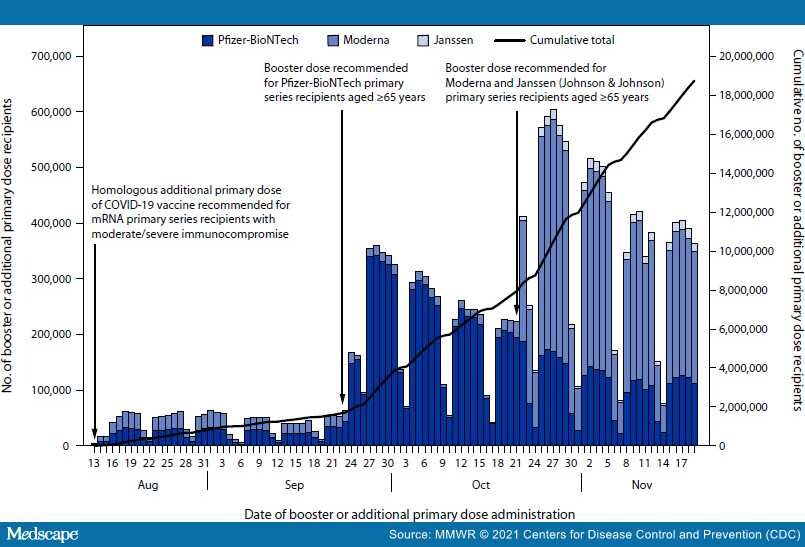
Vaccination against SARS-CoV-2 (the virus that causes COVID-19) is highly effective at preventing hospitalization due to SARS-CoV-2 infection and booster and additional primary dose COVID-19 vaccinations increase protection.[1–3] During August–November 2021, a series of Emergency Use Authorizations and recommendations, including those for an additional primary dose for immunocompromised persons and a booster dose for persons aged ≥18 years, were approved because of reduced immunogenicity in immunocompromised persons, waning vaccine effectiveness over time, and the introduction of the highly transmissible B.1.617.2 (Delta) variant.[4,5] Adults aged ≥65 years are at increased risk for COVID-19–associated hospitalization and death and were one of the populations first recommended a booster dose in the U.S..[5,6] Data on COVID-19 vaccinations reported to CDC from 50 states, the District of Columbia (DC), and eight territories and freely associated states were analyzed to ascertain coverage with booster or additional primary doses among adults aged ≥65 years. During August 13–November 19, 2021, 18.7 million persons aged ≥65 years received a booster or additional primary dose of COVID-19 vaccine, constituting 44.1% of 42.5 million eligible* persons in this age group who previously completed a primary vaccination series.†Coverage was similar by sex and age group, but varied by primary series vaccine product and race and ethnicity, ranging from 30.3% among non-Hispanic American Indian or Alaska Native persons to 50.5% among non-Hispanic multiple/other race persons. Strategic efforts are needed to encourage eligible persons aged ≥18 years, especially those aged ≥65 years and those who are immunocompromised, to receive a booster and/or additional primary dose to ensure maximal protection against COVID-19.