On November 10, 2021, the Michigan Department of Health and Human Services (MDHHS) was notified of a rapid increase in influenza A(H3N2) cases by the University Health Service (UHS) at the University of Michigan in Ann Arbor. Because this outbreak represented some of the first substantial influenza activity during the COVID-19 pandemic, CDC, in collaboration with the university, MDHHS, and local partners conducted an investigation to characterize and help control the outbreak. Beginning August 1, 2021, persons with COVID-19–like* or influenza-like illness evaluated at UHS received testing for SARS-CoV-2, influenza, and respiratory syncytial viruses by rapid multiplex molecular assay.† During October 6–November 19, a total of 745 laboratory-confirmed influenza cases were identified.§ Demographic information, genetic characterization of viruses, and influenza vaccination history data were reviewed. This activity was conducted consistent with applicable federal law and CDC policy.¶
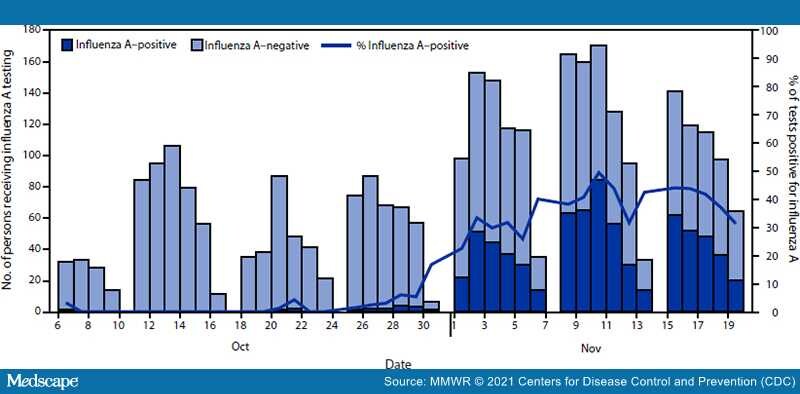
During October 6–November 19, among 3,121 persons tested, 745 (23.9%) received a virus test result that was positive for influenza A, 137 (4.4%) for SARS-CoV-2, and 84 (2.7%) for respiratory syncytial virus. Overall, >95% of influenza cases were detected during November 1–19 (Figure), suggesting rapid spread. One patient with confirmed influenza A infection was hospitalized. Among patients with positive influenza test results, the median age was 19 years (range = 17–31 years), 54.1% were female, 60.0% resided off-campus, 34.6% resided in on-campus residence halls, and 5.4% resided in fraternity or sorority houses. Among 380 specimens sequenced for influenza, all viruses belonged to the A(H3N2) 2a.2 subgroup, which diversified recently from the influenza A(H3N2) subclade 3C.2a1b.2a viruses (i.e., full clade: 3C.2a1b.2a.2). Among 2,405 persons who received testing for influenza A during October 6–November 12, 128 of 481 persons (26.6%) with positive influenza test results and 512 of 1,924 persons (26.6%) with negative influenza test results had documented receipt of 2021–22 influenza vaccine ≥14 days before the test.**