Abstract and Introduction
Introduction
Enterovirus D68 (EV-D68) is associated with a broad spectrum of illnesses, including mild to severe acute respiratory illness (ARI) and acute flaccid myelitis (AFM). Enteroviruses, including EV-D68, are typically detected in the United States during late summer through fall, with year-to-year fluctuations. Before 2014, EV-D68 was infrequently reported to CDC.[1] However, numbers of EV-D68 detection have increased in recent years, with a biennial pattern observed during 2014–2018 in the United States, after the expansion of surveillance and wider availability of molecular testing. In 2014, a national outbreak of EV-D68 was detected.[2] EV-D68 was also reported in 2016 via local[3] and passive national[4] surveillance. EV-D68 detections were limited in 2017, but substantial circulation was observed in 2018.[5] To assess recent levels of circulation, EV-D68 detections in respiratory specimens collected from patients aged <18 years* with ARI evaluated in emergency departments (EDs) or admitted to one of seven U.S. medical centers† within the New Vaccine Surveillance Network (NVSN) were summarized. This report provides a provisional description of EV-D68 detections during July–November in 2018, 2019 and 2020, and describes the demographic and clinical characteristics of these patients. In 2018, a total of 382 EV-D68 detections in respiratory specimens obtained from patients aged <18 years with ARI were reported by NVSN; the number decreased to six detections in 2019 and 30 in 2020. Among patients aged <18 years with EV-D68 in 2020, 22 (73%) were non-Hispanic Black (Black) persons. EV-D68 detections in 2020 were lower than anticipated based on the biennial circulation pattern observed since 2014. The circulation of EV-D68 in 2020 might have been limited by widespread COVID-19 mitigation measures; how these changes in behavior might influence the timing and levels of circulation in future years is unknown. Ongoing monitoring of EV-D68 detections is warranted for preparedness for EV-D68-associated ARI and AFM.
Since 2017, active, population-based, prospective surveillance of EV-D68–associated ARI among patients aged <18 years has been conducted by seven medical institutions in NVSN.§ Respiratory specimens are collected from pediatric patients experiencing ARI (including fever or respiratory symptoms) who are evaluated in EDs or inpatient settings within NVSN. For this study, specimens collected during July–November were tested for EV-D68 using a validated CDC-developed real-time reverse transcription–polymerase chain reaction assay.[5] EV-D68 testing algorithms differed by site.¶ Demographic and clinical data were collected from medical charts or enrollment interviews. This ARI surveillance platform was not designed to capture neurologic outcomes, such as AFM. Detections of EV-D68 in respiratory specimens during July–November in 2018, 2019, and 2020 were assessed by month, site, sex, race/ethnicity, age group, and comorbidities; characteristics were compared by year using univariable chi-square or Wilcoxon rank-sum tests. EV-D68 detections during July–October 2018 have been previously reported.[5] For comparison with 2019 and 2020 data, 2018 data were reanalyzed to include July–November. Available EV-D68–positive specimens from 2020 were submitted to CDC for sequencing. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.**
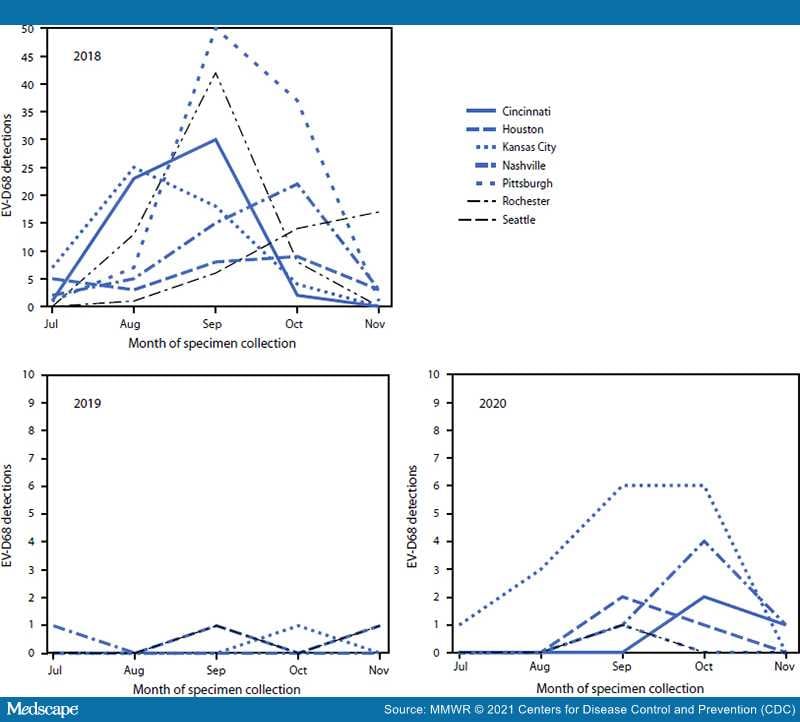
Provisional data from July–November indicated that 3,546 (2018), 3,769 (2019), and 2,189 (2020) patients with ARI were tested for EV-D68 across NVSN. Despite approximately 40% fewer patients aged <18 years being tested during 2020 than in 2018 and 2019, the percentage with a positive rhinovirus or enterovirus (RV/EV) test result remained similar (range = 37.0%–44.2%) (Table 1). Among all patients aged <18 years with ARI tested during July–November, EV-D68 was detected in 382 of 3,546 (10.8%) in 2018, but in only six of 3,769 (0.2%) in 2019 and 30 of 2,189 (1.4%) in 2020; among patients with positive RV/EV test results, EV-D68 was detected in 24.3%, 0.4%, and 3.6% in 2018, 2019, and 2020, respectively. EV-D68 was detected at all seven sites in 2018, at four sites in 2019 and at six sites in 2020 (Figure). During 2018, the highest number of EV-D68 detections occurred in September, and the timing of detections varied by site (Figure); in 2020, October had the highest number of detections. In 2020, 16 of 30 detections (53.3%) occurred in Kansas City, Missouri. Among 23 EV-D68–positive specimens sequenced from 2020, all were clade D.
Figure.
Enterovirus D68 detections, by month and site of specimen collection — New Vaccine Surveillance Network,*,† United States, July–November 2018, 2019, and 2020
Abbreviation: EV-D68 = enterovirus D68.
*The seven sites were in Cincinnati, Ohio; Houston, Texas; Kansas City, Missouri; Nashville, Tennessee; Pittsburgh, Pennsylvania; Rochester, New York; and Seattle, Washington.
†Only sites with EV-D68 detections during that year are shown. During July–November 2019, there were no EV-D68 detections in Cincinnati, Pittsburgh, or Rochester. During July–November 2020, there were no EV-D68 detections in Seattle.
Among 30 patients aged <18 years with EV-D68 in 2020, the median age was 5.3 years, 19 (63.3%) were female, and 15 (50%) required inpatient care (one of whom required mechanical ventilation); none of the patients died (Table 2). Nasal congestion or rhinorrhea, cough, dyspnea, or wheezing were reported in >80% of patients. Asthma or reactive airway disease (RAD) were reported in nearly one half (14; 46.7%) of patients in whom EV-D68 was detected. Compared with the same time frame in 2018, when the median age was 2.9 years and 39.3% of patients with EV-D68–positive respiratory specimens were female, those in 2020 were older (p = 0.04) and more frequently female (p = 0.01).
Among 382 patients with EV-D68–positive specimens in 2018, 53 (13.9%) were Hispanic persons, 125 (32.7%) were non-Hispanic White (White) persons and 161 (42.1%) were Black persons. During the study period in 2019, among six patients with EV-D68–positive specimens, one person was Black and four were Hispanic persons. During the study period in 2020, among 30 patients with EV-D68–positive specimens, three (10.0%) persons were Hispanic, one (3.3%) was White, and 22 (73.3%) were Black. This race/ethnicity distribution was observed during the 2020 study period even after the site in Kansas City, Missouri was excluded, which accounted for approximately one half the cases. In contrast, the race/ethnicity distribution of all patients in NVSN sites with RV/EV was similar across all 3 years, with the proportion of Black persons ranging from 35.0% to 38.3%.
Morbidity and Mortality Weekly Report. 2021;70(47):1623-1628. © 2021 Centers for Disease Control and Prevention (CDC)