This article originally appeared on Hospital for Special Surgery's website.
Case Report
A 39-year-old man with a history of well-controlled type 1 diabetes (T1D) since childhood and migraine presented with 1 week of pain and cyanosis of the fingers of the left hand and toes of both feet. Although he had no history of Raynaud phenomenon, warm temperature yielded transient improvement. He had primary antiphospholipid syndrome (APS), diagnosed 6 years prior on the basis of unprovoked lower-extremity deep vein thrombosis and persistent antiphospholipid antibodies (positive lupus anticoagulant test, high-titer anticardiolipin antibody [aCL] immunoglobulin [Ig] G, and high-titer anti-beta2-glycoprotein I antibody [aβ2GPI] IgG).
He was on warfarin (target international normalized ratio [INR]: 2.5-3), aspirin 81 mg daily, atorvastatin 10 mg daily, and verapamil 240 mg daily. Two weeks prior to presentation, his INR had increased to 7 while on antibiotics for sinusitis; it nadired at 1.3 a week later after warfarin was held.
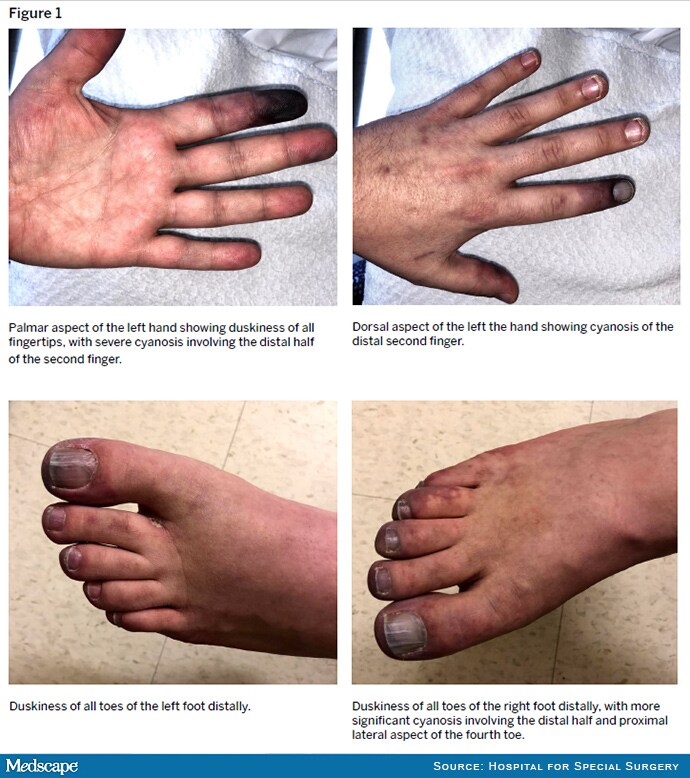
Figure 1.
On physical examination, he had distal cyanosis in the left hand and both feet (Figure 1). Laboratory test results were notable for platelet count of 94 K/μL (normal: 150-450 K/μL), INR of 1.8, one-time troponin elevation to 0.19 ng/mL (normal: ≥ 0.04 ng/mL), and elevated serum transaminases, which peaked at 3 times the upper limit of normal and then trended down over 1 week. Serum levels of complement components 3 and 4 were normal, aCL IgG was > 150 GPL, and aβ2GPI IgG was > 150 SGU. Antineutrophil cytoplasmic antibodies were negative. Glycosylated hemoglobin (A1c) was 6.8% (normal: ≤ 5.6%). Transthoracic and transesophageal echocardiography did not identify valvular vegetations. Magnetic resonance angiography (MRA) of the left hand revealed severe occlusive arterial disease with extensive collateralization (Figure 2).






