Dear Dr. Hahn,
I'm writing because I'm gravely concerned about your leadership of the Food and Drug Administration (FDA). The circumstances of your statements in recent days has led to a crisis in confidence. Not only has your credibility been diminished but so has that of the FDA, its 15,000-plus staff members, and, most importantly, your ability to oversee the health interests of the American people.
Let me remind you of the FDA's mission statement:
"FDA is responsible for advancing the public health by helping to speed innovations that make medical products more effective, safer, and more affordable and by helping the public get the accurate, science-based information they need to use medical products and foods to maintain and improve their health."

Medscape Editor-in-Chief Eric Topol, MD, and FDA Commissioner Stephen Hahn, MD
The emphasis here is on accurate, science-based information. Since you were sworn in on December 17, 2019, you have serially demonstrated your willingness to deviate from this bedrock premise. Immediately after President Trump widely and aggressively promoted hydroxychloroquine as a "miracle drug," on March 30, 2020, you granted an Emergency Use Authorization (EUA) for this drug without any sufficient or meaningful supportive evidence. Proof of that was borne out on June 15, 2020 when you revoked that EUA, acknowledging lack of efficacy and "ongoing serious cardiac adverse events and other potential serious side effects."
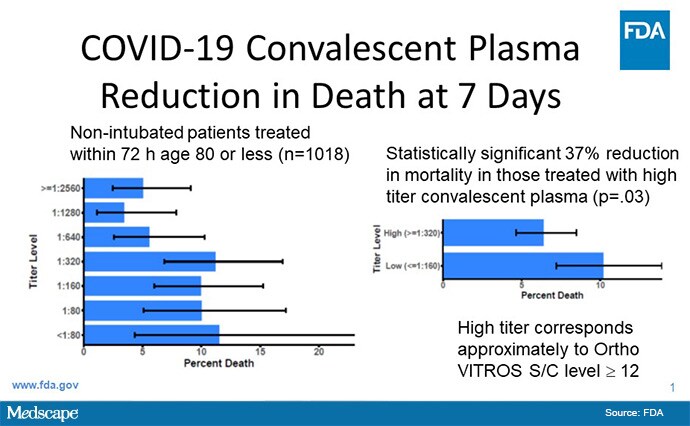
The second major breach of accurate, science-based information came on August 23, 2020 when you participated in a press conference with President Trump and Health and Human Services Secretary Alex Azar billed as a "very historic breakthrough." You said, "I just want to emphasize this point, because I don't want you to gloss over this number. We dream in drug development of something like a 35% mortality reduction. This is a major advance in the treatment of patients. This is a major advance...[A]nd a 35% improvement in survival is a pretty substantial clinical benefit. What that means is — and if the data continue to pan out — [of] 100 people who are sick with COVID-19, 35 would have been saved because of the admission of plasma."
Every part of that statement is incorrect and a blatant misrepresentation of the data. Your statement was based on a preprint, which by definition has not been peer-reviewed, published by Mayo Clinic's Michael Joyner and coauthors. It is a retrospective, observational study of over 35,000 patients who received convalescent plasma, without any controls or untreated patients for comparison. The claim of reduction of mortality is totally unsubstantiated. That was based on improved survival in a subgroup of a subgroup of a subgroup from about 1000 patients, who were partitioned by timing of plasma administration (early vs late), whether they had endotracheal intubation, their age, and level of antibody in the plasma they received. The antibody level was determined post facto. You know full well as an oncologist and researcher that this is an illegitimate analysis that, at best, is hypothesis-generating, requiring a prospective, placebo-controlled trial to confirm.
Nonetheless, you posted this data-dredging subgroup analysis on the FDA website with the headline statement "Another Achievement in Administration's Fight Against Pandemic." Your EUA announcement came the day after President Trump tweeted "The deep state, or whoever, over at the FDA is making it very difficult…@SteveFDA", addressing you directly with your Twitter handle.
It took 24 hours before you started to make a correction on Twitter. You wrote "What I should have said better is that the data show a relative risk reduction, not an absolute reduction."
That is a grossly insufficient correction and does not represent the truth. Here's what you didn't say:
There are no data or evidence from prospective, randomized trials for convalescent plasma to support any survival benefit.
The data I am citing are from a subgroup analysis from a preprint, which is intended to formulate a hypothesis without any definitive findings or conclusions.
The 35% survival benefit, and 35 people's lives saved per 100 sick with COVID-19, was completely off-base. If the preprint data held up in a proper randomized controlled trial, it would be avoiding deaths of 3 or 4 people per 100 who would have died. We know that fewer than 1 out of 100 people who have a COVID-19 infection die, so it is impossible to save 35 people's lives of 100 people sick with COVID-19. I made a terrible, monstrous error and I deeply apologize for that.
It is frankly unlikely for there to be a major survival benefit of convalescent plasma, as it contains a broad admixture of patient antibodies, most of which are not neutralizing — that is having no effect against the virus. We need randomized trials to determine if there is any benefit and, if so, what is the magnitude of benefit. Such trials are ongoing and need robust support and participation.
There are still potential safety issues of convalescent plasma that are unresolved, such as transmission of a virus or immune reaction.
The third breach of evidence-based data was your EUA issued August 28, 2020 broadening the remdesivir approval to include any patient hospitalized with moderate COVID-19. There are insufficient data to support this approval, as it is based on small, open-label studies with subjective endpoints. Remdesivir is an expensive drug, costing approximately $3000 per treatment, in short supply, and even its approval for severe COVID-19 was based on time to recovery in a relatively small trial of just over 1000 patients. That is unlike the proof of dexamethasone benefit for survival in a randomized trial of over 6400 patients.
These repeated breaches demonstrate your willingness to ignore the lack of scientific evidence, and to be complicit with the Trump Administration's politicization of America's healthcare institutions.
In a recent interview with the Financial Times, you said you were prepared to authorize a vaccine before Phase 3 trials were complete. May I remind you that Phase 3 trials are in progress for a few vaccine programs and have only now fulfilled half of their enrollment? It will take many months to establish both safety and efficacy. While most vaccines are safe, trials are needed to demonstrate that participants in these trials do not develop severe immune-mediated reactions to exposure of the virus via antibody-dependent enhancement or immune complex disease. Efficacy data are needed to prove there is a substantial suppression of infections in the vaccine group, compared with placebo. Both safety and efficacy endpoints require adequate statistical power. All of this takes time.
Any shortcuts will not only jeopardize the vaccine programs but betray the public trust, which is already fragile about vaccines, and has been made more so by your lack of autonomy from the Trump administration and its overt politicization of the FDA.
You have one last chance, Dr Hahn, for saving any credibility and preserving trust in the FDA at this critical juncture amidst the pandemic. You need to organize a press conference and tell the truth. Tell Americans exactly how you were pressured to make a breakthrough announcement. Tell all of us how you completely misrepresented the facts about convalescent plasma, and not hide this with the obscurity of technical terms such as relative and absolute differences. Tell us that you are capable and worthy of this pivotal leadership position and that you will not, under any condition, authorize a SARS-CoV-2 vaccine approval before the full Phase 3 completion and read-out of a program.
Otherwise, you need to resign. We cannot entrust the health of 330 million Americans to a person who is subservient to President Trump's whims, unprecedented promotion of unproven therapies, outrageous lies, and political motivations. You have two choices to do the right thing. We cannot and will not rest until you make that choice.
Eric J. Topol, MD, the editor-in-chief of Medscape, is one of the top 10 most cited researchers in medicine and frequently writes about technology in healthcare, including in his latest book, Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again.
For more news, follow Medscape on Facebook, Twitter, Instagram, and YouTube.
Medscape © 2020 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: Eric J. Topol. Dear Commissioner Hahn: Tell the Truth or Resign - Medscape - Aug 31, 2020.












Comments