It's Lyme disease season. Your patient clearly has signs and symptoms that suggest Lyme disease, and you practice in an area where the disease is endemic. So now what do you do? Here are five things you should know before ordering Lyme disease tests.
1. All FDA-cleared Lyme disease tests are serology based.
Serologic tests are designed to detect antibodies made in response to an infection—in this case, infection with Borrelia burgdorferi. Antibodies take a few weeks to develop, so serologic testing might be negative in patients presenting in the first few weeks after infection. Because of this, the clinical usefulness of serologic testing in patients with erythema migrans, the rash of early Lyme disease, might be limited. It's important to remember that patients with erythema migrans from areas where Lyme disease is endemic can be treated empirically with a short course of antibiotics.
Although most healthcare providers would welcome a PCR or culture-based test that would directly detect B burgdorferi infection, the organisms are only briefly present in the bloodstream as they move from the skin to other tissues, so direct detection is usually not possible.
2. IgM and IgG tests can be ordered in the first month of illness. After 30 days, only IgG tests should be ordered.
Lyme disease tests detect both IgM and IgG antibodies. IgM antibodies develop early in disease and can be helpful to diagnose early Lyme disease. However, IgM can be prone to false positives, so if the patient has been ill for more than 30 days, the IgG result alone should be used to diagnose a patient with Lyme disease.
3. CDC currently recommends FDA-cleared Lyme disease tests that use a two-step testing process.
Diagnostic tests that have been FDA-cleared (see FDA's searchable database) are safe and effective and have been evaluated for clinical sensitivity and specificity. Testing for Lyme disease requires a two-step process. If the first step (typically an enzyme immunoassay [EIA]) is negative, no further testing is recommended; the patient's test is negative. If the first step is positive or indeterminate, the second step (traditionally a Western blot) should be performed. The overall result is positive only when the first test is positive (or equivocal) and the second test is positive (or in some cases, equivocal). Do not skip steps in the two-step process. Skipping steps—for example, performing a Western blot alone—increases the chances of a false-positive result.
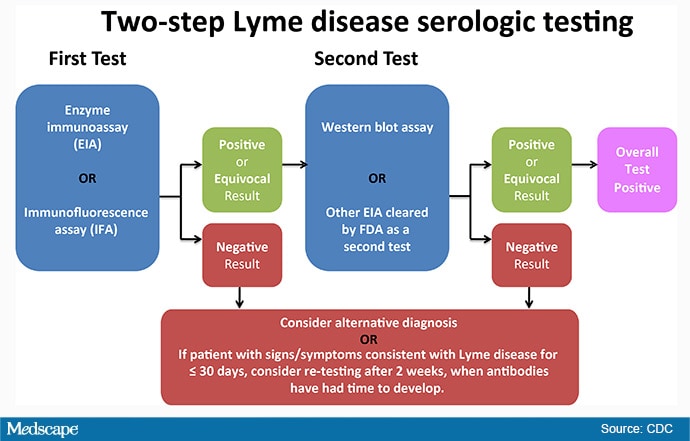
4. In 2019, a new type of two-step test was cleared by the FDA.
The newly cleared two-step algorithm uses a second EIA in place of a Western immunoblot assay for the second step. This alternative approach is referred to as a modified two-tier test. It's important to remember that you can't just use any two EIAs; they need to have been cleared together for this use by the FDA. The new test combination eliminates the subjectivity that has been associated with Western blot interpretation, it is less labor intensive, and it is potentially less costly. Don't worry—the Western blot is still available for use if you prefer that option. If you're not sure about what tests your lab uses, call them to ask.
5. As with previous Lyme disease tests, there is no test of cure.
If your patient tests positive for Lyme disease, they are likely to test positive for months or years, even after effective antibiotic treatment. For this reason, serial testing of patients with Lyme disease is not clinically helpful. Also, don't forget that people who have had Lyme disease can be reinfected if they are exposed to another infected tick. For this reason, it's important to talk to your patients about tick bite prevention when treating them for Lyme disease.
Follow Medscape on Facebook, Twitter, Instagram, and YouTube
Public Information from the CDC and Medscape
Cite this: It's Tick Season: 5 Things to Know About Lyme Disease Tests - Medscape - Apr 06, 2020.










