Palmoplantar psoriasis (PPP) is notoriously hard to treat. PPP presents with erythematous, scaling plaques, fissures, and sterile pustules on the palms and soles that cause significant pain and morbidity. Risk factors include a genetic predisposition, smoking, and female sex.
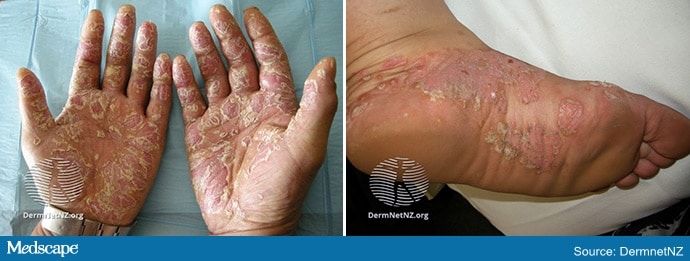
Management is difficult, with results that are often disappointing and unpredictable. Traditional treatments—ultrapotent topical corticosteroids, oral retinoids, methotrexate, cyclosporine, and phototherapy (UVB or PUVA)—typically only lead to partial improvement.
The IL-23/17 proinflammatory cytokine pathway is important in the pathogenesis of both psoriasis vulgaris and PPP. Guselkumab, a humanized monoclonal antibody to the p19 protein subunit of IL-23, has been shown to be very effective in clearing moderate to severe chronic plaque psoriasis when compared with placebo and the TNF-alpha blocker adalimumab. A small pilot study of this agent conducted in Japanese patients with PPP also found promising results.
Spurred on by these encouraging findings, the Japanese investigators launched a phase 3, randomized, crossover trial to confirm the safety and efficacy of guselkumab as a monotherapy for PPP.
This larger study enrolled 159 Japanese patients with PPP (mean age, 53.3 years; 79.2% female). Participants were randomly assigned to receive placebo, guselkumab 100 mg, or guselkumab 200 mg subcutaneously at weeks 0, 4, 12, and then every 8 weeks for a total of 60 weeks. At week 16, those in the placebo group crossed over to receive guselkumab.
Results. By week 16, disease scores as measured by objective tools had improved more for patients in both guselkumab groups compared with placebo. Over half of those in the guselkumab 100 mg group had experienced more than 50% improvement compared with about a third of those on placebo. Surprisingly, however, patients in the 200 mg guselkumab group had reductions in severity of disease comparable to those seen with placebo. The self-reported disease severity was improved more for patients on both guselkumab doses. Improvements continued through week 52. Adverse events in both guselkumab groups were comparable to those of placebo. This 60-week study confirmed that guselkumab is a safe and modestly effective treatment for PPP.
Viewpoint
PPP is a pain in the neck to treat. Even patients with a limited body surface area of involvement tend to be miserable. Affecting the palms and soles, the disease causes a disproportionate amount of pain and morbidity, often preventing patients from working with their hands while limiting mobility. This is compounded by the refractory nature of PPP. Topical therapies (corticosteroids, calcipotriene, tazarotene) yield modest improvement at best, and responses tend to wane over time. Even systemic therapies (oral retinoids, methotrexate, cyclosporine, apremilast) are hit-or-miss, with patients often proving refractory to combination regimens.
This study examining the safety and efficacy of guselkumab is welcome. While results suggest that guselkumab is far from a home run, it shows promise even as a monotherapy. It would be interesting to see whether better results can be achieved in combination with other therapies, such as topical corticosteroids.
One important caveat: This study was conducted in a Japanese population, so future studies need to explore whether these results hold true for other populations. The study also had a crossover design at week 16, so a placebo-to-guselkumab comparison could not be made beyond week 16; therefore, long-term efficacy is impossible to assess.
Finally, more patients in the guselkumab 100 mg group achieved 50% improvement than in the guselkumab 200 mg group. The investigators suggested that this may be due to disproportionately worse disease in the 200 mg group, but future studies will need to clarify this discrepancy.
In sum, guselkumab may not be the silver bullet we've all been hoping for to treat PPP, but at least it may give some relief to those suffering from this miserable form of psoriasis.
Graeme Lipper is a clinical assistant professor at the University of Vermont Medical College and a partner at Advanced Dermcare in Danbury, Connecticut.
Follow Medscape on Facebook, Twitter, Instagram, and YouTube
Medscape Dermatology © 2019 WebMD, LLC
Any views expressed above are the author's own and do not necessarily reflect the views of WebMD or Medscape.
Cite this: Graeme M. Lipper. Palmoplantar Psoriasis: Does a New Drug Offer Some Relief? - Medscape - Nov 20, 2019.

Comments