Tramadol hydrochloride (ConZip™); Tramadol hydrochloride (Ultram®); Acetaminophen; tramadol hydrochloride (Ultracet®); Tramadol hydrochloride (Ultram® ER)
For more on this Drug Safety Labeling Change, click here for Tramadol hydrochloride (ConZip™) ; Tramadol hydrochloride (Ultram®); Acetaminophen; tramadol hydrochloride (Ultracet®); and Tramadol hydrochloride (Ultram® ER).
For full prescribing information, click here and here.
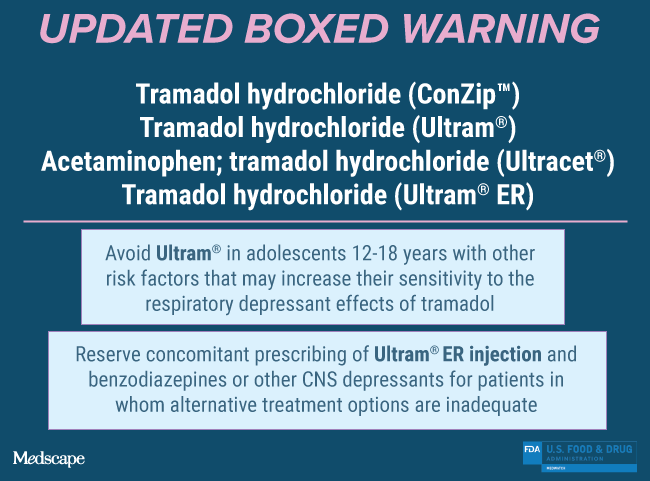
Updated Boxed Warning
WARNING: ADDICTION, ABUSE, AND MISUSE; LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL INGESTION; ULTRA-RAPID METABOLISM OF TRAMADOL AND OTHER RISK FACTORS FOR LIFE-THREATENING RESPIRATORY DEPRESSION IN CHILDREN; NEONATAL OPIOID WITHDRAWAL SYNDROME; INTERACTIONS WITH DRUGS AFFECTING CYTOCHROME P450 ISOENZYMES; and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Ultra-Rapid Metabolism of Tramadol and Other Risk Factors for Life-Threatening Respiratory Depression in Children
Life-threatening respiratory depression and death have occurred in children who received tramadol. Some of the reported cases followed tonsillectomy and/or adenoidectomy; in at least one case, the child had evidence of being an ultra-rapid metabolizer of tramadol due to a CYP2D6 polymorphism (see WARNINGS). ULTRAM is contraindicated in children younger than 12 years of age and in children younger than 18 years of age following tonsillectomy and/or adenoidectomy (see CONTRAINDICATIONS). Avoid the use of ULTRAM in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of tramadol.
Updated Boxed Warning: Tramadol hydrochloride (Ultram® ER)
WARNING: ADDICTION, ABUSE, AND MISUSE; LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL INGESTION; ULTRA-RAPID METABOLISM OF TRAMADOL AND OTHER RISK FACTORS FOR LIFE-THREATENING RESPIRATORY DEPRESSION IN CHILDREN; NEONATAL OPIOID WITHDRAWAL SYNDROME; INTERACTIONS WITH DRUGS AFFECTING CYTOCHROME P450 ISOENZYMES; and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Ultra-Rapid Metabolism of Tramadol and Other Risk Factors for Life-Threatening Respiratory Depression in Children
Life-threatening respiratory depression and death have occurred in children who received tramadol. Some of the reported cases followed tonsillectomy and/or adenoidectomy; in at least one case, the child had evidence of being an ultra-rapid metabolizer of tramadol due to a CYP2D6 polymorphism. ULTRAM ER is contraindicated in children younger than 12 years of age and in children younger than 18 years of age following tonsillectomy and/or adenoidectomy. Avoid the use of ULTRAM ER in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of tramadol.
Risks From Concomitant Use With Benzodiazepines or Other CNS Depressants
Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death.
Reserve concomitant prescribing of ULTRAM ER injection and benzodiazepines or other CNS depressants for use in patients for whom alternative treatment options are inadequate.
Limit dosages and durations to the minimum required.
Follow patients for signs and symptoms of respiratory depression and sedation.
Public Information from the FDA and Medscape
Information provided by FDA and/or its employees on this website is for educational purposes only, and does not constitute medical advice. Any statement or advice given by an FDA employee on this website does not represent the formal position of FDA. FDA and/or any FDA employee will not be liable for injury or other damages resulting to any individuals who view FDA-related materials on this website.
Cite this: Drug Safety Warnings and Updates: July-September 2017 - Medscape - Jan 03, 2018.

