Codeine phosphate and chlorpheniramine maleate (TUXARIN ER™)
For more on this Drug Safety Labeling Change, click here.
For full prescribing information, click here.
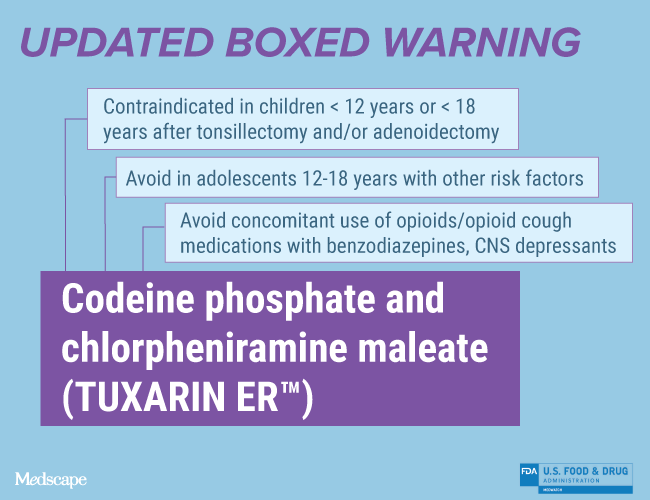
Updated Boxed Warning
ULTRA-RAPID METABOLISM OF CODEINE AND OTHER RISK FACTORS FOR LIFE-THREATENING RESPIRATORY DEPRESSION IN CHILDREN and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS
Ultra-Rapid Metabolism of Codeine and Other Risk Factors for Life-Threatening Respiratory Depression in Children
Life-threatening respiratory depression and death have occurred in children who received codeine; most cases followed tonsillectomy and/or adenoidectomy, and many of the children had evidence of being an ultra-rapid metabolizer of codeine due to a CYP2D6 polymorphism. TUXARIN ER is contraindicated in children younger than 12 years of age and in children younger than 18 years of age following tonsillectomy and/or adenoidectomy. Avoid the use of TUXARIN ER in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of codeine.
Concomitant Use With Benzodiazepines, CNS Depressants
Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Avoid use of opioid cough medications in patients taking benzodiazepines, other CNS depressants, or alcohol.
Chlorpheniramine polistirex; codeine polistirex (Tuzistra® XR)
For more on this Drug Safety Labeling Change, click here.
For full prescribing information, click here.

Updated Boxed Warning
ULTRA-RAPID METABOLISM OF CODEINE AND OTHER RISK FACTORS FOR LIFE-THREATENING RESPIRATORY DEPRESSION IN CHILDREN
Life-threatening respiratory depression and death have occurred in children who received codeine. Most of the reported cases occurred following tonsillectomy and/or adenoidectomy, and many of the children had evidence of being an ultra-rapid metabolizer of codeine due to a CYP2D6 polymorphism. Tuzistra XR is contraindicated in children younger than 12 years of age and in children younger than 18 years of age following tonsillectomy and/or adenoidectomy. Avoid the use of TUZISTRA XR in adolescents 12 to 18 years of age who have other risk factors that may increase their sensitivity to the respiratory depressant effects of codeine.
RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS ...
Public Information from the FDA and Medscape
Information provided by FDA and/or its employees on this website is for educational purposes only, and does not constitute medical advice. Any statement or advice given by an FDA employee on this website does not represent the formal position of FDA. FDA and/or any FDA employee will not be liable for injury or other damages resulting to any individuals who view FDA-related materials on this website.
Cite this: Drug Safety Warnings and Updates: July-September 2017 - Medscape - Jan 03, 2018.









