United States Multicenter Clinical Trial of Corneal Collagen Crosslinking for Keratoconus Treatment
Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK; United States Crosslinking Study Group Ophthalmology. 2017 May 7. [Epub ahead of print]
Study Summary
Corneal collagen crosslinking (CXL), a procedure to strengthen the cornea originally devised to treat patients with keratoconus, is now also used to treat post-refractive surgery ectasia. It was developed in Europe over 15 years ago and has been in widespread use around the world for at least the past 5 years. It was approved by the US Food and Drug Administration (FDA) in April 2016.
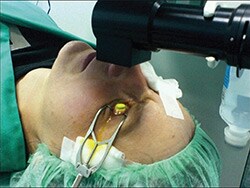
Hersh and colleagues report on the results of the trial used to obtain the FDA approval, which included patients aged 14 years or older with progressive keratoconus and thinnest corneal thickness greater than 300 microns, measured by Pentacam. The technique entailed removing the epithelium, placing riboflavin for 30 minutes, and checking the pachymetry. When the pachymetry was less than 400 microns (which was probably fairly common, as the inclusion criteria allowed corneal thicknesses down to 300 microns prior to epithelial removal), additional hypotonic riboflavin was placed until the thickness reached at least 400 microns. UVA light was then applied for 30 minutes.
Patients were randomized to treatment or sham groups, with the latter receiving 30 minutes of riboflavin drops but no epithelial removal and no UVA light.








